This article focuses on the antimicrobial mechanism of Gemini Surfactants, which are expected to be effective in killing bacteria and can provide some help in slowing down the spread of new coronaviruses.
Surfactant, which is a contraction of the phrases Surface, Active and Agent. Surfactants are substances that are active on surfaces and interfaces and have a very high ability and efficiency in reducing surface (boundary) tension, forming molecularly ordered assemblies in solutions above a certain concentration and thus having a range of application functions. Surfactants possess good dispersibility, wettability, emulsification ability, and antistatic properties, and have become key materials for the development of many fields, including the field of fine chemicals, and have a significant contribution in improving processes, reducing energy consumption, and increasing production efficiency. With the development of society and the continuous progress of the world's industrial level, the application of surfactants has gradually spread from daily-use chemicals to various fields of the national economy, such as antibacterial agents, food additives, new energy fields, pollutant treatment and biopharmaceuticals.
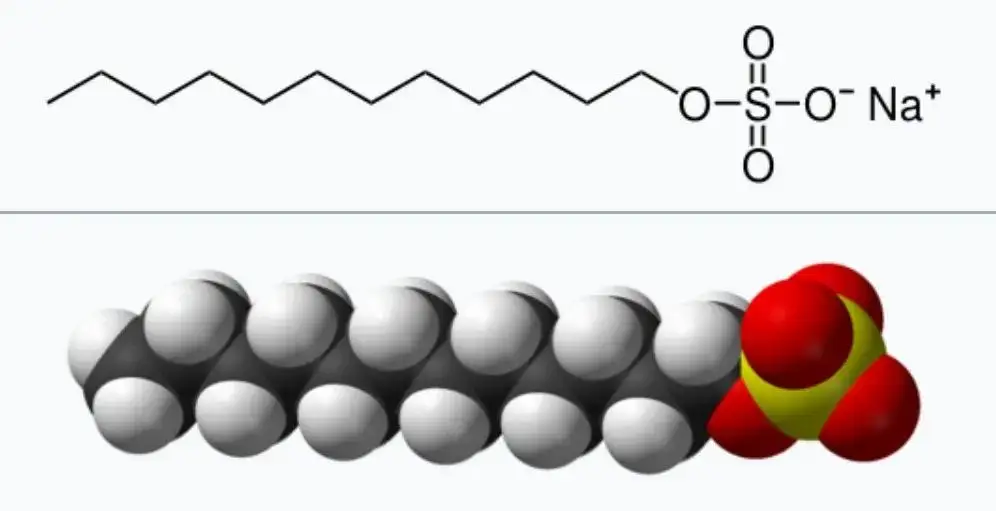
Conventional surfactants are "amphiphilic" compounds consisting of polar hydrophilic groups and nonpolar hydrophobic groups, and their molecular structures are shown in Figure 1(a).
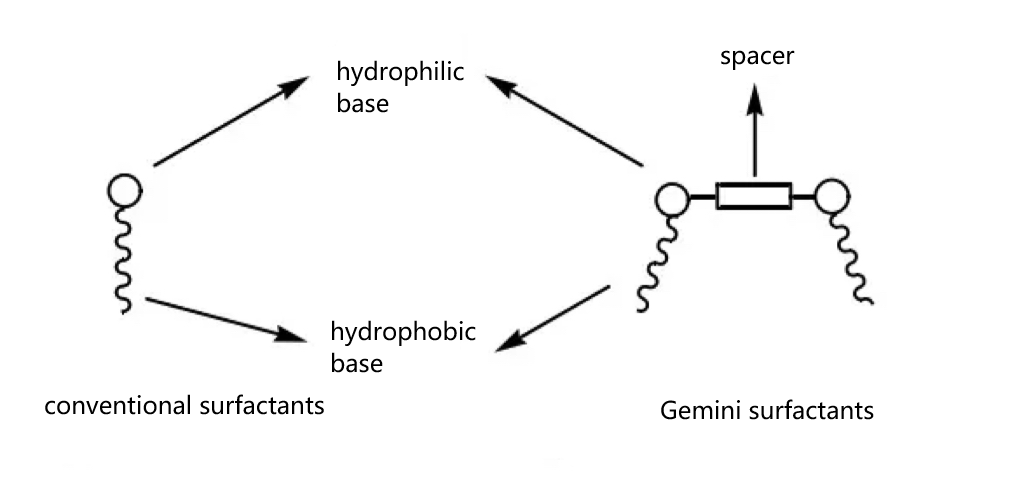
At present, with the development of refinement and systematization in the manufacturing industry, the demand for surfactant properties in the production process is gradually increasing, so it is important to find and develop surfactants with higher surface properties and with special structures. The discovery of Gemini Surfactants bridges these gaps and meets the requirements of industrial production. A common Gemini surfactant is a compound with two hydrophilic groups (generally ionic or nonionic with hydrophilic properties) and two hydrophobic alkyl chains.
As shown in Figure 1(b), in contrast to conventional single-chain surfactants, Gemini Surfactants link two hydrophilic groups together through a linking group (spacer). In short, the structure of a Gemini surfactant can be understood as formed by cleverly bonding two hydrophilic head groups of a conventional surfactant together with a linkage group.
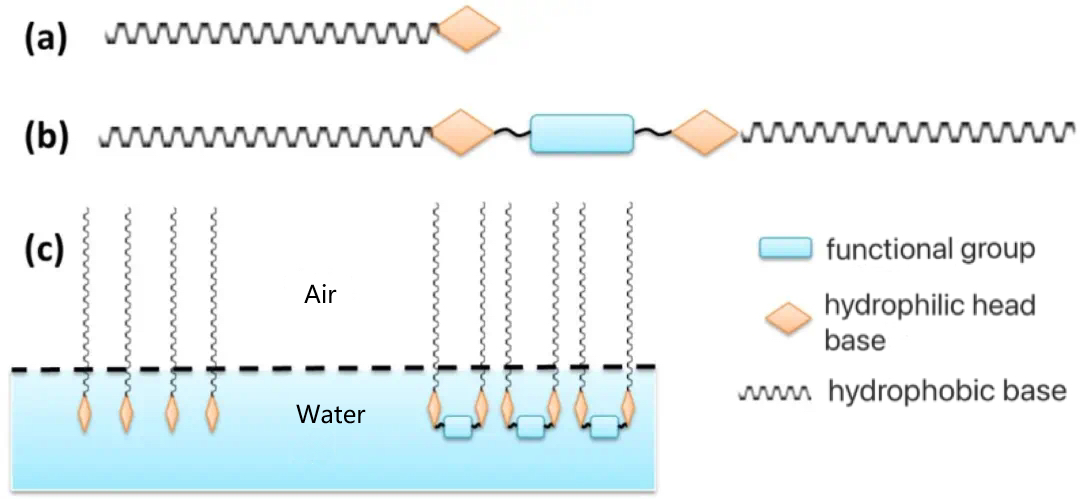
The special structure of the Gemini Surfactant leads to its high surface activity, which is mainly due to:
(1) the enhanced hydrophobic effect of the two hydrophobic tail chains of the Gemini Surfactant molecule and the increased tendency of the surfactant to leave the aqueous solution.
(2) The tendency of hydrophilic head groups to separate from each other, especially ionic head groups due to electrostatic repulsion, is substantially weakened by the influence of spacer;
(3) The special structure of Gemini Surfactants affects their aggregation behavior in aqueous solution, giving them a more complex and variable aggregation morphology.
Gemini Surfactants have higher surface (boundary) activity, lower critical micelle concentration, better wettability, emulsification ability and antibacterial ability compared with conventional surfactants. Therefore, the development and utilization of Gemini Surfactants are of great significance for the development and application of surfactants.
The "amphiphilic structure" of conventional surfactants gives them unique surface properties. As shown in Figure 1(c), when a conventional surfactant is added to water, the hydrophilic head group tends to dissolve inside the aqueous solution, and the hydrophobic group inhibits the dissolution of the surfactant molecule in water. Under the combined effect of these two trends, the surfactant molecules are enriched at the gas-liquid interface and undergo an orderly arrangement, thereby reducing the surface tension of water. Unlike conventional surfactants, Gemini Surfactants are "dimers" that link conventional surfactants together through spacer groups, which can reduce the surface tension of water and oil/water interfacial tension more effectively. In addition, Gemini Surfactants have lower critical micelle concentrations, better water solubility, emulsification, foaming, wetting and antibacterial properties.
| Introduction of Gemini Surfactants In 1991, Menger and Littau [13] prepared the first bis-alkyl chain surfactant with a rigid linkage group, and named it "Gemini surfactant". In the same year, Zana et al [14] prepared a series of quaternary ammonium salt Gemini Surfactants for the first time and systematically investigated the properties of this series of quaternary ammonium salt Gemini Surfactants. 1996, researchers generalized and discussed the surface (boundary) behavior, aggregation properties, solution rheology and phase behavior of different Gemini Surfactants when compounded with conventional surfactants. In 2002, Zana [15] investigated the effect of different linkage groups on the aggregation behavior of Gemini Surfactants in aqueous solution, a work that greatly advanced the development of surfactants and was of great significance. Later, Qiu et al [16] invented a new method for the synthesis of Gemini Surfactants containing special structures based on cetyl bromide and 4-amino-3,5-dihydroxymethyl-1,2,4-triazole, which further enriched the way of Gemini Surfactant synthesis. |
Research on Gemini Surfactants in China started late; in 1999, Jianxi Zhao from Fuzhou University made a systematic review of foreign research on Gemini Surfactants and attracted the attention of many research institutions in China. After that, the research on Gemini Surfactants in China began to flourish and achieved fruitful results. In recent years, researchers have devoted themselves to the development of new Gemini Surfactants and the study of their related physicochemical properties. At the same time, the applications of Gemini Surfactants have been gradually developed in the fields of sterilization and antibacterial, food production, defoaming and foam inhibition, drug slow release and industrial cleaning. Based on whether the hydrophilic groups in surfactant molecules are charged or not and the type of charge they carry, Gemini Surfactants can be divided into the following categories: cationic, anionic, nonionic and amphoteric Gemini Surfactants. Among them, cationic Gemini Surfactants generally refer to quaternary ammonium or ammonium salt Gemini Surfactants, anionic Gemini Surfactants mostly refer to Gemini Surfactants whose hydrophilic groups are sulfonic acid, phosphate and carboxylic acid, while nonionic Gemini Surfactants are mostly polyoxyethylene Gemini Surfactants.
1.1 Cationic Gemini Surfactants
Cationic Gemini Surfactants can dissociate cations in aqueous solutions, mainly ammonium and quaternary ammonium salt Gemini Surfactants. Cationic Gemini Surfactants have good biodegradability, strong decontamination ability, stable chemical properties, low toxicity, simple structure, easy synthesis, easy separation and purification, and also have bactericidal properties, anticorrosion, antistatic properties and softness.
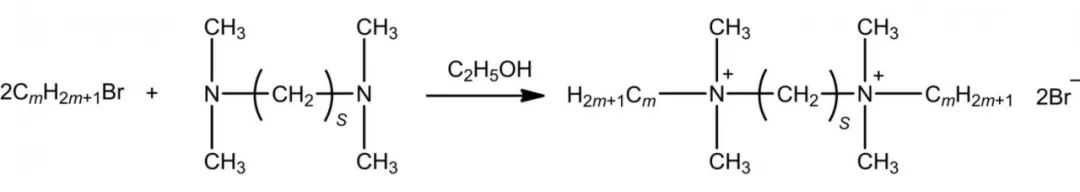
Quaternary ammonium salt-based Gemini Surfactants are generally prepared from tertiary amines by alkylation reactions. There are two main synthetic methods as follows: one is to quaternize dibromo-substituted alkanes and single long-chain alkyl dimethyl tertiary amines; the other is to quaternize 1-bromo-substituted long-chain alkanes and N,N,N',N'-tetramethyl alkyl diamines with anhydrous ethanol as solvent and heating reflux. However, dibromo-substituted alkanes are more expensive and are commonly synthesized by the second method, and the reaction equation is shown in Figure 2.
1.2 Anionic Gemini Surfactants
Anionic Gemini Surfactants can dissociate anions in aqueous solution, mainly sulfonates, sulfate salts, carboxylates and phosphate salts type Gemini Surfactants. Anionic surfactants have better properties such as decontamination, foaming, dispersion, emulsification and wetting, and are widely used as detergents, foaming agents, wetting agents, emulsifiers and dispersants.
1.2.1 Sulfonates
Sulfonate-based biosurfactants have the advantages of good water solubility, good wettability, good temperature and salt resistance, good detergency, and strong dispersing ability, and they are widely used as detergents, foaming agents, wetting agents, emulsifiers, and dispersants in petroleum, textile industry, and daily-use chemicals because of their relatively wide sources of raw materials, simple production processes, and low costs. Li et al synthesized a series of new dialkyl disulfonic acid Gemini Surfactants (2Cn-SCT), a typical sulfonate-type baryonic surfactant, using trichloramine, aliphatic amine and taurine as raw materials in a three-step reaction.
1.2.2 Sulfate salts
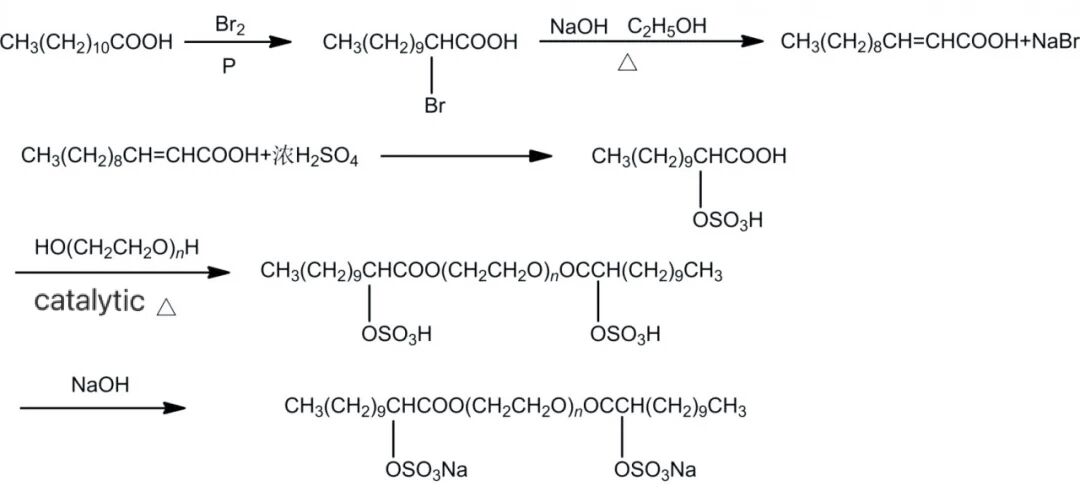
Sulfate ester salts doublet surfactants have the advantages of ultra-low surface tension, high surface activity, good water solubility, wide source of raw materials and relatively simple synthesis. It also has good washing performance and foaming ability, stable performance in hard water, and sulfate ester salts are neutral or slightly alkaline in aqueous solution. As shown in Figure 3, Sun Dong et al used lauric acid and polyethylene glycol as the main raw materials and added sulfate ester bonds through substitution, esterification and addition reactions, thus synthesizing the sulfate ester salt type baryonic surfactant-GA12-S-12.
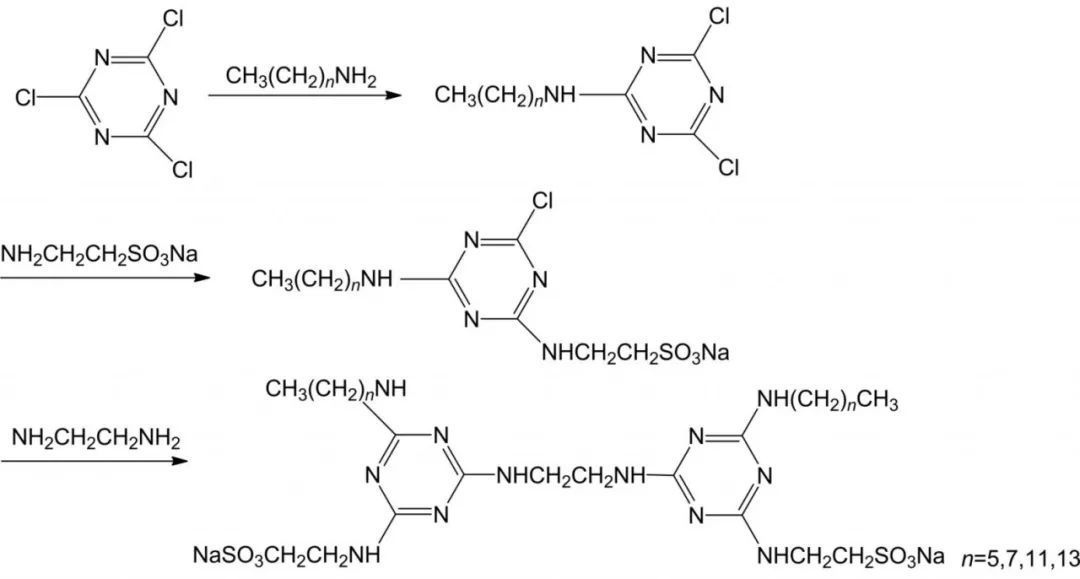
1.2.3 Carboxylic acid salts
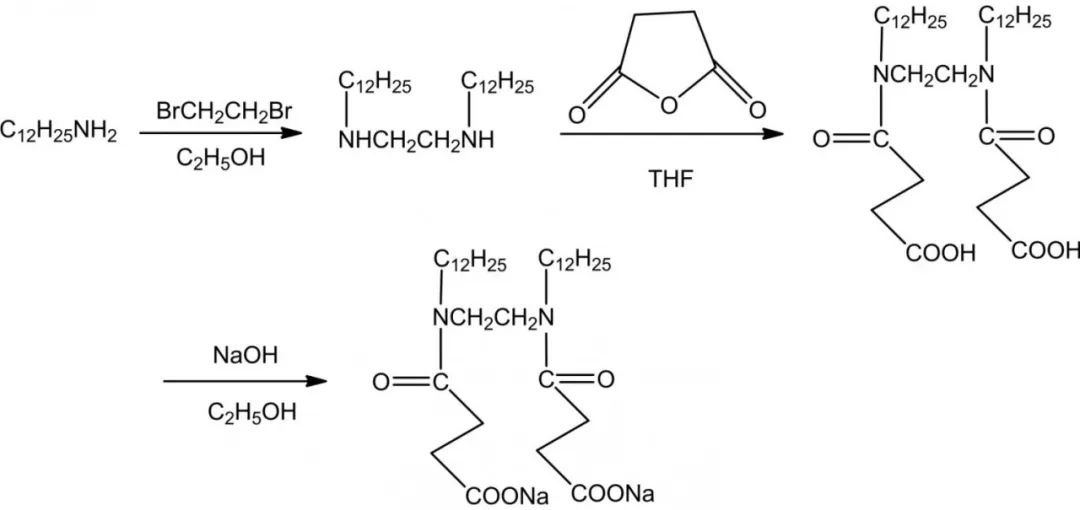
Carboxylate-based Gemini Surfactants are usually mild, green, easily biodegradable and have a rich source of natural raw materials, high metal chelating properties, good hard water resistance and calcium soap dispersion, good foaming and wetting properties, and are widely used in pharmaceuticals, textiles, fine chemicals and other fields. The introduction of amide groups in carboxylate-based biosurfactants can enhance the biodegradability of surfactant molecules and also make them have good wetting, emulsification, dispersion and decontamination properties. Mei et al synthesized a carboxylate-based baryonic surfactant CGS-2 containing amide groups using dodecylamine, dibromoethane and succinic anhydride as raw materials.
1.2.4 Phosphate salts
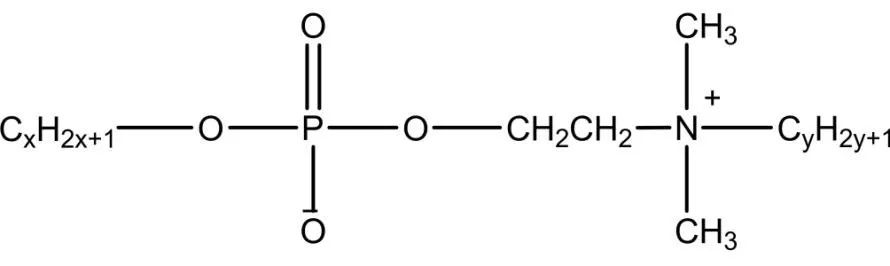
Phosphate ester salt type Gemini Surfactants have a similar structure to natural phospholipids and are prone to form structures such as reverse micelles and vesicles. Phosphate ester salt type Gemini Surfactants have been widely used as antistatic agents and laundry detergents, while their high emulsification properties and relatively low irritation have led to their wide use in personal skin care. Certain phosphate esters can be anticancer, antitumor and antibacterial, and dozens of drugs have been developed. Phosphate ester salt type biosurfactants have high emulsification properties for pesticides and can be used not only as antibacterial and insecticides but also as herbicides. Zheng et al studied the synthesis of phosphate ester salt Gemini Surfactants from P2O5 and ortho-quat-based oligomeric diols, which have better wetting effect, good antistatic properties, and a relatively simple synthesis process with mild reaction conditions. The molecular formula of the potassium phosphate salt baryonic surfactant is shown in Figure 4.
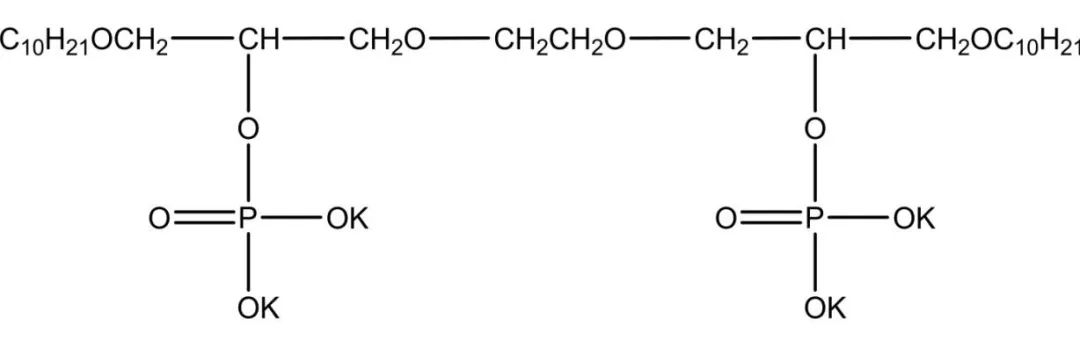
1.3 Non-ionic Gemini Surfactants
Nonionic Gemini Surfactants cannot be dissociated in aqueous solution and exist in molecular form. This type of baryonic surfactant has been less studied so far, and there are two types, one is a sugar derivative and the other is alcohol ether and phenol ether. Nonionic Gemini Surfactants do not exist in the ionic state in solution, so they have high stability, are not easily affected by strong electrolytes, have good complexability with other types of surfactants, and have good solubility. Therefore, nonionic surfactants have various properties such as good detergency, dispersibility, emulsification, foaming, wettability, antistatic property and sterilization, and can be widely used in various aspects such as pesticides and coatings. As shown in Figure 5, in 2004, FitzGerald et al synthesized polyoxyethylene based Gemini Surfactants (nonionic surfactants), whose structure was expressed as (Cn-2H2n-3CHCH2O(CH2CH2O)mH)2(CH2)6 (or GemnEm).
02 Physicochemical properties of Gemini Surfactants
2.1 Activity of Gemini Surfactants
The simplest and most direct way to evaluate the surface activity of surfactants is to measure the surface tension of their aqueous solutions. In principle, surfactants reduce the surface tension of a solution by oriented arrangement on the surface (boundary) plane (Figure 1(c)). The critical micelle concentration (CMC) of Gemini Surfactants is more than two orders of magnitude smaller and the C20 value is significantly lower compared to conventional surfactants with similar structures. The baryonic surfactant molecule possesses two hydrophilic groups that help it maintain good water solubility while having long hydrophobic long chains. At the water/air interface, the conventional surfactants are loosely arranged due to the spatial site resistance effect and the repulsion of homogeneous charges in the molecules, thus weakening their ability to reduce the surface tension of water. In contrast, the linking groups of Gemini Surfactants are covalently bonded so that the distance between the two hydrophilic groups is kept within a small range (much smaller than the distance between the hydrophilic groups of conventional surfactants), resulting in better activity of Gemini Surfactants at the surface (boundary).
2.2 Assembly structure of Gemini Surfactants
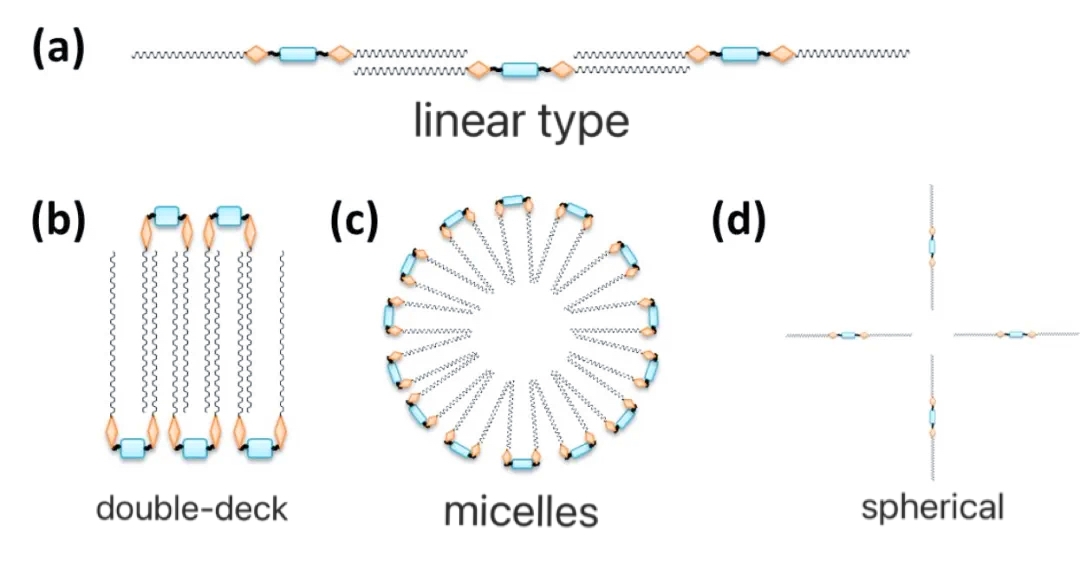
In aqueous solutions, as the concentration of baryonic surfactant increases, its molecules saturate the surface of the solution, which in turn forces other molecules to migrate to the interior of the solution to form micelles. The concentration at which the surfactant starts to form micelles is called Critical Micelle Concentration (CMC). As shown in Figure 9, after the concentration is greater than CMC, unlike conventional surfactants that aggregate to form spherical micelles, Gemini Surfactants produce a variety of micelle morphologies, such as linear and bilayer structures, because of their structural characteristics. The differences in micelle size, shape and hydration have a direct impact on the phase behavior and rheological properties of the solution, and also lead to changes in solution viscoelasticity. Conventional surfactants, such as anionic surfactants (SDS), usually form spherical micelles, which have almost no effect on the viscosity of the solution. However, the special structure of Gemini Surfactants leads to the formation of more complex micelle morphology and the properties of their aqueous solutions differ significantly from those of conventional surfactants. The viscosity of aqueous solutions of Gemini Surfactants increases with increasing concentration of Gemini Surfactants, probably because the formed linear micelles intertwine into a web-like structure. However, the viscosity of the solution decreases with increasing surfactant concentration, probably due to the disruption of the web structure and the formation of other micelle structures.
03 Antimicrobial properties of Gemini Surfactants
As a kind of organic antimicrobial agent, the antimicrobial mechanism of baryonic surfactant is mainly that it combines with anions on the cell membrane surface of microorganisms or reacts with sulfhydryl groups to disrupt the production of their proteins and cell membranes, thus destroying microbial tissues to inhibit or kill microorganisms.
3.1 Antimicrobial properties of anionic Gemini Surfactants
The antimicrobial properties of antimicrobial anionic surfactants are mainly determined by the nature of the antimicrobial moieties they carry. In colloidal solutions such as natural latexes and coatings, hydrophilic chains bind to water-soluble dispersants, and hydrophobic chains will bind to hydrophobic dispersions by directional adsorption, thus transforming the two-phase interface into a dense molecular interfacial film. The bacterial inhibitory groups on this dense protective layer inhibit the growth of bacteria.
The mechanism of bacterial inhibition of anionic surfactants is fundamentally different from that of cationic surfactants. The bacterial inhibition of anionic surfactants is related to their solution system and the inhibition groups, so this type of surfactant can be limited. This type of surfactant must be present at sufficient levels so that the surfactant is present in every corner of the system to produce a good microbicidal effect. At the same time, this type of surfactant lacks localization and targeting, which not only causes unnecessary waste, but also creates resistance over a long period of time.
As an example, alkyl sulfonate-based biosurfactants have been used in clinical medicine. Alkyl sulfonates, such as Busulfan and Treosulfan, mainly treat myeloproliferative diseases, acting to produce cross-linking between guanine and ureapurine, while this alteration cannot be repaired by cellular proofreading, resulting in apoptotic cell death.
3.2 Antimicrobial properties of cationic Gemini Surfactants
The main type of cationic Gemini Surfactants developed is quaternary ammonium salt type Gemini Surfactants. Quaternary ammonium type cationic Gemini Surfactants have strong bactericidal effect because there are two hydrophobic long alkane chains in quaternary ammonium type baryonic surfactant molecules, and the hydrophobic chains form hydrophobic adsorption with the cell wall (peptidoglycan); at the same time, they contain two positively charged nitrogen ions, which will promote the adsorption of surfactant molecules to the surface of negatively charged bacteria, and through penetration and diffusion, the hydrophobic chains penetrate deeply into the Bacterial cell membrane lipid layer, change the permeability of the cell membrane, leading to the rupture of the bacterium, in addition to hydrophilic groups deep into the protein, leading to the loss of enzyme activity and protein denaturation, due to the combined effect of these two effects, making the fungicide has a strong bactericidal effect.
However, from an environmental point of view, these surfactants have hemolytic activity and cytotoxicity, and longer contact time with aquatic organisms and biodegradation can increase their toxicity.
3.3 Antibacterial properties of nonionic Gemini Surfactants
There are currently two types of nonionic Gemini Surfactants, one is a sugar derivative and the other is alcohol ether and phenol ether.
The antibacterial mechanism of sugar-derived biosurfactants is based on the affinity of the molecules, and sugar-derived surfactants can bind to cell membranes, which contain a large number of phospholipids. When the concentration of sugar derivatives surfactants reaches a certain level, it changes the permeability of the cell membrane, forming pores and ion channels, which affects the transport of nutrients and gas exchange, causing the outflow of contents and eventually leading to the death of the bacterium.
The antibacterial mechanism of phenolic and alcoholic ethers antimicrobial agents is to act on the cell wall or cell membrane and enzymes, blocking metabolic functions and disrupting regenerative functions. For example, antimicrobial drugs of diphenyl ethers and their derivatives (phenols) are immersed in bacterial or viral cells and act through the cell wall and cell membrane, inhibiting the action and function of enzymes related to the synthesis of nucleic acids and proteins, limiting the growth and reproduction of bacteria. It also paralyzes the metabolic and respiratory functions of the enzymes within the bacteria, which then fail.
3.4 Antibacterial properties of amphoteric Gemini Surfactants
Amphoteric Gemini Surfactants are a class of surfactants that have both cations and anions in their molecular structure, can ionize in aqueous solution, and exhibit the properties of anionic surfactants in one medium condition and cationic surfactants in another medium condition. The mechanism of bacterial inhibition of amphoteric surfactants is inconclusive, but it is generally believed that the inhibition may be similar to that of quaternary ammonium surfactants, where the surfactant is easily adsorbed on the negatively charged bacterial surface and interferes with bacterial metabolism.
3.4.1 Antimicrobial properties of amino acid Gemini Surfactants
Amino acid type baryonic surfactant is a cationic amphoteric baryonic surfactant composed of two amino acids, so its antimicrobial mechanism is more similar to that of quaternary ammonium salt type baryonic surfactant. The positively charged part of the surfactant is attracted to the negatively charged part of the bacterial or viral surface due to electrostatic interaction, and subsequently the hydrophobic chains bind to the lipid bilayer, leading to efflux of cell contents and lysis until death. It has significant advantages over quaternary ammonium-based Gemini Surfactants: easy biodegradability, low hemolytic activity, and low toxicity, so it is being developed for its application and its field of application is being expanded.
3.4.2 Antibacterial properties of non-amino acid type Gemini Surfactants
The non-amino acid type amphoteric Gemini Surfactants have surface active molecular residues containing both non-ionizable positive and negative charge centers. The main non-amino acid type Gemini Surfactants are betaine, imidazoline, and amine oxide. Taking betaine type as an example, betaine-type amphoteric surfactants have both anionic and cationic groups in their molecules, which are not easily affected by inorganic salts and have surfactant effects in both acidic and alkaline solutions, and the antimicrobial mechanism of cationic Gemini Surfactants is followed in acidic solutions and that of anionic Gemini Surfactants in alkaline solutions. It also has excellent compounding performance with other types of surfactants.
04 Conclusion and outlook
Gemini Surfactants are increasingly used in life because of their special structure, and they are widely used in the fields of antibacterial sterilization, food production, defoaming and foam inhibition, drug slow release and industrial cleaning. With the increasing demand for green environment protection, Gemini Surfactants are gradually developed into environmentally friendly and multifunctional surfactants. Future research on Gemini Surfactants can be carried out in the following aspects: developing new Gemini Surfactants with special structures and functions, especially strengthening the research on antibacterial and antiviral; compounding with common surfactants or additives to form products with better performance; and using cheap and easily available raw materials to synthesize environmentally friendly Gemini Surfactants.
Post time: Mar-25-2022

