

The shrinkage force of any unit length on the surface of the liquid is called the surface tension, and the unit is N.·m-1.

The property of reducing the surface tension of the solvent is called surface activity, and a substance with this property is called a surface-active substance.
The surface-active substance that can bind molecules in aqueous solution and form micelles and other associations, and have high surface activity, while also having the effect of wetting, emulsifying, foaming, washing, etc. is called surfactant.
Surfactant is organic compounds with special structure and property, which can significantly change the interfacial tension between two phases or the surface tension of liquids (generally water), with wetting, foaming, emulsifying, washing and other properties.
In terms of structure, surfactants have a common feature in that they contain two groups of different nature in their molecules. At one end is a long chain of non-polar group, soluble in oil and insoluble in water, also known as hydrophobic group or water-repellent group. Such water-repellent group is generally long chains of hydrocarbons, sometimes also for organic fluorine, silicon, organophosphate, organotin chain, etc. At the other end is water-soluble group, a hydrophilic group or oil-repellent group. The hydrophilic group must be sufficiently hydrophilic to ensure that entire surfactants are soluble in water and has the necessary solubility. Since surfactants contain hydrophilic and hydrophobic groups, they can be soluble in at least one of the liquid phases. This hydrophilic and lipophilic property of surfactant is called amphiphilicity.


Surfactant is a kind of amphiphilic molecules with both hydrophobic and hydrophilic groups. Hydrophobic groups of surfactants are generally composed of long-chain hydrocarbons, such as straight-chain alkyl C8~C20, branched-chain alkyl C8~C20,alkylphenyl (alkyl carbon tom number is 8~16) and the like. The difference which is small between hydrophobic groups is mainly in the structural changes of hydrocarbon chains. And the types of hydrophilic groups are more, so the properties of surfactants are mainly related to hydrophilic groups in addition to the size and shape of hydrophobic groups. The structural changes of hydrophilic groups are larger than those of hydrophobic groups, so the classification of surfactants is generally based on the structure of hydrophilic groups. This classification is based on whether the hydrophilic group is ionic or not, and it is divided into anionic, cationic, nonionic, zwitterionic and other special types of surfactants.

① Adsorption of surfactants at the interfac
Surfactant molecules are amphiphilic molecules having both lipophilic and hydrophilic groups. When the surfactant is dissolved in water, its hydrophilic group is attracted to water and dissolves in water, while its lipophilic group is repelled by water and leaves water, resulting in the adsorption of surfactant molecules (or ions) on the interface of the two phases, which reduces the interfacial tension between the two phases. The more surfactant molecules (or ions) are adsorbed at the interface, the greater the reduction in interfacial tension.
② Some properties of adsorption membrane
Surface pressure of adsorption membrane: Surfactant adsorption at the gas-liquid interface to form an adsorption membrane, such as place a frictionless removable floating sheet on the interface, the floating sheet pushes the adsorbent membrane along the solution surface, and the membrane generates a pressure on the floating sheet, which is called surface pressure.
Surface viscosity: Like surface pressure, surface viscosity is a property exhibited by insoluble molecular membrane. Suspended by a fine metal wire platinum ring, so that its plane contacts the water surface of the tank, rotate the platinum ring, the platinum ring by the viscosity of the water hindrance, the amplitude gradually decay, according to which the surface viscosity can be measured. The method is: first, the experiment is conducted on the pure water surface to measure the amplitude decay, and then the decay after the formation of the surface membrane is measured, and the viscosity of the surface membrane is derived from the difference between the two.
The surface viscosity is closely related to the solidity of the surface membrane, and since the adsorption membrane has surface pressure and viscosity, it must have elasticity. The higher the surface pressure and the higher the viscosity of the adsorbed membrane, the higher its elastic modulus. The elastic modulus of the surface adsorption membrane is important in the process of bubble stabilization.
③ Formation of micelles
Dilute solutions of surfactants obey the laws followed by ideal solutions. The amount of surfactant adsorbed on the surface of the solution increases with the concentration of the solution, and when the concentration reaches or exceeds a certain value, the amount of adsorption no longer increases, and these excess surfactant molecules are in the solution in a haphazard way or in some regular way. Both practice and theory show that they form associations in solution, and these associations are called micelles.
Critical Micelle Concentration (CMC): The minimum concentration at which surfactants form micelles in solution is called the critical micelle concentration.
④ CMC values of common surfactants.

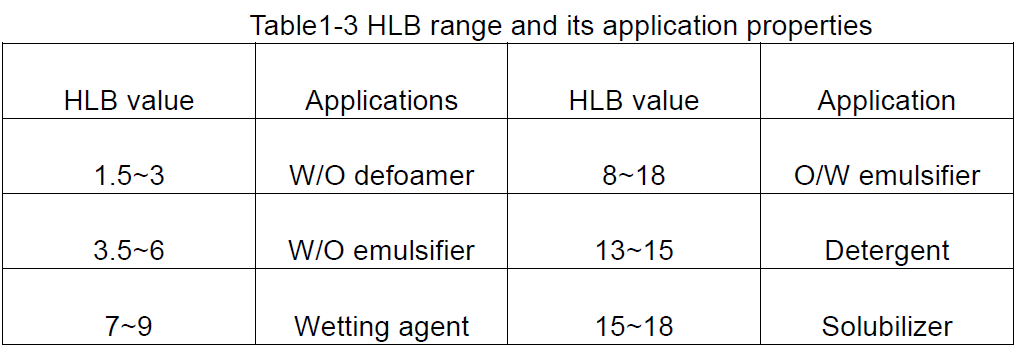
HLB is the abbreviation of hydrophile lipophile balance, which indicates the hydrophilic and lipophilic balance of the hydrophilic and lipophilic groups of the surfactant, i.e., the HLB value of the surfactant. A large HLB value indicates a molecule with strong hydrophilicity and weak lipophilicity; conversely, strong lipophilicity and weak hydrophilicity.
① Provisions of HLB value
The HLB value is a relative value, so when the HLB value is developed, as a standard, the HLB value of paraffin wax, which has no hydrophilic properties, is specified to be 0, while the HLB value of sodium dodecyl sulfate, which is more water-soluble, is 40. Therefore, the HLB value of surfactants is generally within the range of 1 to 40. Generally speaking, emulsifiers with HLB values less than 10 are lipophilic, while those greater than 10 are hydrophilic. Thus, the turning point from lipophilic to hydrophilic is about 10.
Based on the HLB values of surfactants, a general idea of their possible uses can be obtained, as shown in Table 1-3.

Two mutually insoluble liquids, one dispersed in the other as particles (droplets or liquid crystals) form a system called an emulsion. This system is thermodynamically unstable due to the increase in the boundary area of the two liquids when the emulsion is formed. In order to make the emulsion stable, it is necessary to add a third component - emulsifier to reduce the interfacial energy of the system. Emulsifier belongs to surfactant, its main function is to play the role of emulsion. The phase of the emulsion that exists as droplets is called the dispersed phase (or inner phase, discontinuous phase), and the other phase that is linked together is called the dispersion medium (or outer phase, continuous phase).
① Emulsifiers and emulsions
Common emulsions, one phase is water or aqueous solution, the other phase is organic substances not miscible with water, such as grease, wax, etc. The emulsion formed by water and oil can be divided into two types according to their dispersion situation: oil dispersed in water to form oil-in-water type emulsion, expressed as O/W (oil/water): water dispersed in oil to form oil-in-water type emulsion, expressed as W/O (water/oil). Complex water-in-oil-in-water W/O/W type and oil-in-water-in-oil O/W/O type multi-emulsions may also be formed.
Emulsifiers are used to stabilize emulsions by reducing interfacial tension and forming single-molecule interfacial membrane.
In the emulsification of the emulsifier requirements:
a: The emulsifier must be able to adsorb or enrich the interface between the two phases, so that the interfacial tension is reduced;
b: The emulsifier must give the particles to the charge, so that electrostatic repulsion between the particles, or forms a stable, highly viscous protective membrane around the particles.
Therefore, the substance used as an emulsifier must have amphiphilic groups in order to emulsify, and surfactants can meet this requirement.
② Preparation methods of emulsions and factors affecting the stability of emulsions
There are two ways to prepare emulsions: one is to use the mechanical method to disperse the liquid in tiny particles in another liquid, which is mostly used in industry to prepare emulsions; the other is to dissolve the liquid in molecular state in another liquid, and then make it gather properly to form emulsions.
The stability of an emulsion is the ability to anti-particle aggregation that leads to phase separation. Emulsions are thermodynamically unstable systems with large free energy. Therefore, the so-called stability of an emulsion is actually the time required for the system to reach equilibrium, i.e., the time required for separation of one of the liquids in the system to occur.
When the interfacial membrane with fatty alcohols, fatty acids and fatty amines and other polar organic molecules, membrane strength significantly higher. This is because, in the interfacial adsorption layer of emulsifier molecules and alcohols, acids and amines and other polar molecules to form a "complex", so that the interfacial membrane strength increased.
Emulsifiers consisting of more than two surfactants are called mixed emulsifiers. Mixed emulsifier adsorbed at the water/oil interface; intermolecular action can form complexes. Due to the strong intermolecular action, the interfacial tension is significantly reduced, the amount of emulsifier adsorbed at the interface is significantly increased, the formation of interfacial membrane density increases, the strength increases.
The charge of the liquid beads has a significant effect on the stability of the emulsion. Stable emulsions, whose liquid beads are generally charged. When an ionic emulsifier is used, the emulsifier ion adsorbed at the interface has its lipophilic group inserted into the oil phase and the hydrophilic group is in the water phase, thus making the liquid beads charged. As the emulsion beads with the same charge, they repel each other, not easy to agglomerate, so that the stability is increased. It can be seen that the more emulsifier ions adsorbed on the beads, the greater the charge, the greater the ability to prevent the beads from agglomeration, the more stable the emulsion system.
The viscosity of the emulsion dispersion medium has a certain influence on the stability of the emulsion. Generally, the higher the viscosity of the dispersion medium, the higher the stability of the emulsion. This is because the viscosity of the dispersion medium is large, which has a strong effect on the Brownian motion of the liquid beads and slows down the collision between the liquid beads, so that the system remains stable. Usually, the polymer substances that can be dissolved in emulsions can increase the viscosity of the system and make the stability of emulsions higher. In addition, polymers can also form a strong interfacial membrane, making the emulsion system more stable.
In some cases, the addition of solid powder can also make the emulsion tends to stabilize. Solid powder is in the water, oil or interface, depending on the oil, water on the wetting capacity of the solid powder, if the solid powder is not completely wet with water, but also wet by oil, will remain on the water and oil interface.
The solid powder does not make the emulsion stable because the powder gathered at the interface enhances the interfacial membrane, which is similar to the interfacial adsorption of emulsifier molecules, so the more closely the solid powder material is arranged at the interface, the more stable the emulsion is.
Surfactants have the ability to significantly increase the solubility of insoluble or slightly water-soluble organic substances after forming micelles in aqueous solution, and the solution is transparent at this time. This effect of the micelle is called solubilization. The surfactant that can produce solubilization is called solubilizer, and the organic matter that is solubilized is called solubilized matter.

Foam plays an important role in the washing process. Foam is a dispersion system in which a gas is dispersed in a liquid or solid, with the gas as the dispersed phase and the liquid or solid as the dispersing medium, the former being called liquid foam, while the latter is called solid foam, such as foamed plastic, foamed glass, foamed cement etc.
(1) Foam formation
By foam we mean here an aggregate of air bubbles separated by a liquid membrane. This type of bubble always rises quickly to the liquid surface due to the large difference in density between the dispersed phase (gas) and the dispersion medium (liquid), combined with the low viscosity of the liquid.
The process of forming a bubble is to bring a large amount of gas into the liquid, and the bubbles in the liquid quickly return to the surface, forming an aggregate of bubbles separated by a small amount of liquid gas.
Foam has two significant characteristics in terms of morphology: one is that the bubbles as a dispersed phase are often polyhedral in shape, this is because at the intersection of the bubbles, there is a tendency for the liquid film to thin so that the bubbles become polyhedral, when the liquid film thins to a certain extent, it leads to bubble rupture; the second is that pure liquids cannot form stable foam, the liquid that can form foam is at least two or more components. Aqueous solutions of surfactants are typical of systems that are prone to foam generation, and their ability to generate foam is also related to other properties.
Surfactants with good foaming power are called foaming agents. Although the foaming agent has good foam ability, but the foam formed may not be able to maintain a long time, that is, its stability is not necessarily good. In order to maintain the stability of the foam, often in the foaming agent to add substances that can increase the stability of the foam, the substance is called foam stabilizer, commonly used stabilizer is lauryl diethanolamine and dodecyl dimethylamine oxide.
(2) Stability of the foam
Foam is a thermodynamically unstable system and the final trend is that the total surface area of the liquid within the system decreases after the bubble is broken and the free energy decreases. The defoaming process is the process by which the liquid membrane separating the gas becomes thicker and thinner until it breaks. Therefore, the degree of stability of the foam is mainly determined by the speed of liquid discharge and the strength of the liquid film. The following factors also influence this.
(3) Foam destruction
The basic principle of foam destruction is to change the conditions that produce the foam or to eliminate the stabilizing factors of the foam, thus there are both physical and chemical methods of defoaming.
Physical defoaming means changing the conditions of foam production while maintaining the chemical composition of the foam solution, such as external disturbances, changes in temperature or pressure and ultrasonic treatment are all effective physical methods to eliminate foam.
The chemical defoaming method is to add certain substances to interact with the foaming agent to reduce the strength of the liquid film in the foam and thus reduce the stability of the foam to achieve the purpose of defoaming, such substances are called defoamers. Most of the defoamers are surfactants. Therefore, according to the mechanism of defoaming, defoamer should have a strong ability to reduce surface tension, easy to adsorb on the surface, and the interaction between the surface adsorption molecules is weak, adsorption molecules arranged in a more loosen structure.
There are various types of defoamer, but basically, they are all non-ionic surfactants. Non-ionic surfactants have anti-foaming properties near or above their cloud point and are often used as defoamers. Alcohols, especially alcohols with a branching structure, fatty acids and fatty acid esters, polyamides, phosphate esters, silicone oils, etc. are also commonly used as excellent defoamers.
(4) Foam and washing
There is no direct link between foam and washing effectiveness and the amount of foam does not indicate the effectiveness of the wash. For example, nonionic surfactants have far fewer foaming properties than soaps, but their decontamination is much better than soaps.
In some cases, foam can be helpful in removing dirt and grime. For example, when washing dishes in the home, the foam of the detergent picks up the oil droplets and when scrubbing carpets, the foam helps to pick up dust, powder and other solid dirt. In addition, foam can sometimes be used as an indication of the effectiveness of a detergent. Because fatty oils have an inhibiting effect on the foam of the detergent, when there is too much oil and too little detergent, no foam will be generated or the original foam will disappear. Foam can also sometimes be used as an indicator of the cleanliness of a rinse, as the amount of foam in the rinse solution tends to decrease with the reduction of detergent, so the amount of foam can be used to evaluate the degree of rinsing.
In a broad sense, washing is the process of removing unwanted components from the object to be washed and achieving some purpose. Washing in the usual sense refers to the process of removing dirt from the surface of the carrier. In washing, the interaction between the dirt and the carrier is weakened or eliminated by the action of some chemical substances (e.g., detergent, etc.), so that the combination of dirt and carrier is changed into the combination of dirt and detergent, and finally the dirt is separated from the carrier. As the objects to be washed and the dirt to be removed are diverse, washing is a very complex process and the basic process of washing can be expressed in the following simple relationships.
Carrie··Dirt + Detergent= Carrier + Dirt·Detergent
The washing process can usually be divided into two stages: firstly, under the action of the detergent, the dirt is separated from its carrier; secondly, the detached dirt is dispersed and suspended in the medium. The washing process is a reversible process and the dirt dispersed and suspended in the medium may also be re-precipitated from the medium to the object being washed. Therefore, a good detergent should have the ability to disperse and suspend dirt and prevent redeposition of dirt, in addition to the ability to remove dirt from the carrier.
(1) Types of dirt
Even for the same item, the type, composition and amount of dirt can vary depending on the environment in which it is used. Oil body dirt is mainly some animal and vegetable oils and mineral oils (such as crude oil, fuel oil, coal tar, etc.), solid dirt is mainly soot, ash, rust, carbon black, etc. In terms of clothing dirt, there is dirt from the human body, such as sweat, sebum, blood, etc.; dirt from food, such as fruit stains, cooking oil stains, condiment stains, starch, etc.; dirt from cosmetics, such as lipstick, nail polish, etc.; dirt from the atmosphere, such as soot, dust, mud, etc.; others, such as ink, tea, coating, etc. It comes in various types.
The various types of dirt can usually be divided into three main categories: solid dirt, liquid dirt and special dirt.
① Solid dirt
Common solid dirt includes particles of ash, mud, earth, rust and carbon black. Most of these particles have an electrical charge on their surface, most of them are negatively charged and can be easily adsorbed on fiber items. Solid dirt is generally difficult to dissolve in water, but can be dispersed and suspended by detergent solutions. Solid dirt with smaller mass point is more difficult to remove.
② Liquid dirt
Liquid dirt is mostly oil-soluble, including plant and animal oils, fatty acids, fatty alcohols, mineral oils and their oxides. Among them, plant and animal oils, fatty acids and alkali saponification can occur, while fatty alcohols, mineral oils are not saponified by alkali, but can be soluble in alcohols, ethers and hydrocarbon organic solvents, and detergent water solution emulsification and dispersion. Oil-soluble liquid dirt generally has a strong force with fiber items, and is more firmly adsorbed on fibers.
③ Special dirt
Special dirt includes proteins, starch, blood, human secretions such as sweat, sebum, urine and fruit juice and tea juice. Most of this type of dirt can be chemically and strongly adsorbed on fiber items. Therefore, it is difficult to wash.
The various types of dirt are rarely found alone, but are often mixed together and adsorbed onto the object. Dirt can sometimes be oxidized, decomposed or decayed under external influences, thus creating new dirt.
(2)Adhesion of dirt
Clothes, hands etc. can be stained because there is some kind of interaction between the object and the dirt. Dirt adheres to objects in a variety of ways, but there are no more than physical and chemical adhesions.
①The adhesion of soot, dust, mud, sand and charcoal to clothing is a physical adhesion. Generally speaking, through this adhesion of dirt, and the role between the stained object is relatively weak, the removal of dirt is also relatively easy. According to the different forces, the physical adhesion of dirt can be divided into mechanical adhesion and electrostatic adhesion.
A: Mechanical adhesion
This type of adhesion mainly refers to the adhesion of some solid dirt (e.g., dust, mud and sand). Mechanical adhesion is one of the weaker forms of adhesion of dirt and can be removed almost by purely mechanical means, but when the dirt is small (<0.1um), it is more difficult to remove.
B:Electrostatic adhesion
Electrostatic adhesion is mainly manifested in the action of charged dirt particles on oppositely charged objects. Most fibrous objects are negatively charged in water and can easily be adhered to by certain positively charged dirt, such as lime types. Some dirt, although negatively charged, such as carbon black particles in aqueous solutions, can adhere to fibers through ionic bridges (ions between multiple oppositely charged objects, acting together with them in a bridge-like manner) formed by positive ions in water (e.g., Ca2+, Mg2+ etc.).
Electrostatic action is stronger than simple mechanical action, making dirt removal relatively difficult.
② Chemical adhesion
Chemical adhesion refers to the phenomenon of dirt acting on an object through chemical or hydrogen bonds. For example, polar solid dirt, protein, rust and other adhesion on fiber items, fibers contain carboxyl, hydroxyl, amide and other groups, these groups and oily dirt fatty acids, fatty alcohols are easy to form hydrogen bonds. The chemical forces are generally strong and the dirt is therefore more firmly bonded to the object. This type of dirt is difficult to remove by the usual methods and requires special methods to deal with it.
The degree of adhesion of dirt is related to the nature of the dirt itself and the nature of the object to which it is adhered. Generally, particles adhere easily to fibrous items. The smaller the texture of the solid dirt, the stronger the adhesion. Polar dirt on hydrophilic objects such as cotton and glass adhere more strongly than non-polar dirt. Non-polar dirt adheres more strongly than polar dirt, such as polar fats, dust and clay, and is less easy to remove and clean.
(3) Dirt removal mechanism
The purpose of washing is to remove dirt. In a medium of a certain temperature (mainly water). Using the various physical and chemical effects of the detergent to weaken or eliminate the effect of dirt and washed objects, under the action of certain mechanical forces (such as hand rubbing, washing machine agitation, water impact), so that the dirt and washed objects from the purpose of decontamination.
① Mechanism of liquid dirt removal
A:Wetting
Liquid soiling is mostly oil-based. Oil stains wet most fibrous items and spread more or less as an oil film on the surface of the fibrous material. The first step in the washing action is the wetting of the surface by the washing liquid. For the sake of illustration, the surface of a fiber can be thought of as a smooth solid surface.
B: Oil detachment - curling mechanism
The second step in the washing action is the removal of oil and grease, the removal of liquid dirt is achieved by a kind of coiling. The liquid dirt originally existed on the surface in the form of a spread oil film, and under the preferential wetting effect of the washing liquid on the solid surface (i.e., the fiber surface), it curled up into oil beads step by step, which were replaced by the washing liquid and eventually left the surface under certain external forces.
② Mechanism of solid dirt removal
The removal of liquid dirt is mainly through the preferential wetting of the dirt carrier by the washing solution, while the removal mechanism for solid dirt is different, where the washing process is mainly about the wetting of the dirt mass and its carrier surface by the washing solution. Due to the adsorption of surfactants on the solid dirt and its carrier surface, the interaction between the dirt and the surface is reduced and the adhesion strength of the dirt mass on the surface is reduced, thus the dirt mass is easily removed from the surface of the carrier.
In addition, the adsorption of surfactants, especially ionic surfactants, on the surface of the solid dirt and its carrier has the potential to increase the surface potential on the surface of the solid dirt and its carrier, which is more conducive to the removal of the dirt. Solid or generally fibrous surfaces are usually negatively charged in aqueous media and can therefore form diffuse double electronic layers on dirt masses or solid surfaces. Due to the repulsion of homogeneous charges, the adhesion of dirt particles in the water to the solid surface is weakened. When an anionic surfactant is added, because it can simultaneously increase the negative surface potential of the dirt particle and the solid surface, the repulsion between them is more enhanced, the adhesion strength of the particle is more reduced, and the dirt is easier to remove.
Non-ionic surfactants are adsorbed on generally charged solid surfaces and although they do not significantly change the interfacial potential, the adsorbed non-ionic surfactants tend to form a certain thickness of adsorbed layer on the surface which helps to prevent redeposition of dirt.
In the case of cationic surfactants, their adsorption reduces or eliminates the negative surface potential of the dirt mass and its carrier surface, which reduces the repulsion between the dirt and the surface and is therefore not conducive to dirt removal; furthermore, after adsorption on the solid surface, cationic surfactants tend to turn the solid surface hydrophobic and are therefore not conducive to surface wetting and therefore washing.
③ Removal of special soils
Protein, starch, human secretions, fruit juice, tea juice and other such dirt are difficult to remove with normal surfactants and require special treatment.
Protein stains such as cream, eggs, blood, milk and skin excreta tend to coagulate on the fibers and degeneration and get stronger adhesion. Protein soiling can be removed by using proteases. The enzyme protease breaks down the proteins in the dirt into water-soluble amino acids or oligopeptides.
Starch stains mainly come from foodstuffs, others such as gravy, glue etc. Amylase has a catalytic effect on the hydrolysis of starch stains, causing starch to break down into sugars.
Lipase catalyzes the decomposition of triglycerides, which are difficult to remove by normal methods, such as sebum and edible oils, and breaks them down into soluble glycerol and fatty acids.
Some colored stains from fruit juices, tea juices, inks, lipstick etc. are often difficult to clean thoroughly even after repeated washing. These stains can be removed by a redox reaction with an oxidizing or reducing agent such as bleach, which destroys the structure of the color-generating or color-auxiliary groups and degrades them into smaller water-soluble components.
(4)Stain removal mechanism of dry cleaning
The above is actually for water as the medium of washing. In fact, due to the different types of clothing and structure, some clothing using water washing is not convenient or not easy to wash clean, some clothing after washing and even deformation, fading, etc., for example: most natural fibers absorb water and easy to swell, and dry and easy to shrink, so after washing will be deformed; by washing wool products also often appear shrinkage phenomenon, some woolen products with water washing is also easy to pilling, color change; Some silks hand feeling turns worse after washing and lose their luster. For these clothes often use the dry-cleaning method to decontaminate. The so-called dry cleaning generally refers to the washing method in organic solvents, especially in non-polar solvents.
Dry cleaning is a gentler form of washing than water washing. Because dry cleaning does not require much mechanical action, it does not cause damage, wrinkling and deformation to clothing, while dry cleaning agents, unlike water, rarely produce expansion and contraction. As long as the technology is properly handled, the clothes can be dry cleaned without distortion, color fading and extended service life.
In terms of dry cleaning, there are three broad types of dirt.
①Oil-soluble dirt Oil-soluble dirt includes all kinds of oil and grease, which is liquid or greasy and can be dissolved in dry cleaning solvents.
②Water-soluble dirt Water-soluble dirt is soluble in aqueous solutions, but not in dry cleaning agents, is adsorbed on clothing in an aqueous state, water evaporates after the precipitation of granular solids, such as inorganic salts, starch, protein, etc.
③Oil and water insoluble dirt Oil and water insoluble dirt is neither soluble in water nor soluble in dry cleaning solvents, such as carbon black, silicates of various metals and oxides , etc.
Due to the different nature of various types of dirt, there are different ways of removing dirt in the dry-cleaning process. Oil-soluble soils, such as animal and vegetable oils, mineral oils and greases, are easily soluble in organic solvents and can be removed more easily in dry cleaning. The excellent solubility of dry-cleaning solvents for oils and greases essentially comes from the van der Walls forces between molecules.
For the removal of water-soluble dirt such as inorganic salts, sugars, proteins and sweat, the right amount of water must also be added to the dry-cleaning agent, otherwise water-soluble dirt is difficult to remove from the clothing. However, water is difficult to dissolve in the dry-cleaning agent, so to increase the amount of water, you also need to add surfactants. The presence of water in the dry-cleaning agent can make the surface of the dirt and clothing hydrated, so that it is easy to interact with the polar groups of surfactants, which is conducive to the adsorption of surfactants on the surface. In addition, when surfactants form micelles, water-soluble dirt and water can be solubilized into the micelles. In addition to increasing the water content of the dry-cleaning solvent, surfactants can also play a role in preventing the re-deposition of dirt to enhance the decontamination effect.
The presence of a small amount of water is necessary to remove water-soluble dirt, but too much water can cause distortion and wrinkling in some clothes, so the amount of water in the dry-cleaning agent must be moderate.
Dirt that is neither water-soluble nor oil-soluble, solid particles like ash, mud, earth and carbon black, is generally attached to the garment by electrostatic forces or in combination with oil. In dry cleaning, the flow of solvent, impact can make the electrostatic force adsorption of dirt off, and dry-cleaning agent can dissolve the oil, so that the combination of oil and dirt and attached to the clothing of solid particles off in the dry-cleaning agent, dry cleaning agent in a small amount of water and surfactants, so that those off the solid dirt particles can be stable suspension, dispersion, to prevent its re-deposition to the clothing.
(5)Factors affecting washing action
The directional adsorption of surfactants at the interface and the reduction of surface (interfacial) tension are the main factors in the removal of liquid or solid dirt. However, the washing process is complex and the washing effect, even with the same detergent type, is influenced by many other factors. These factors include the concentration of the detergent, the temperature, the nature of the soiling, the type of fiber and the structure of the fabric.
① Surfactant concentration
The micelles of surfactants in solution play an important role in the washing process. When the concentration reaches the critical micelle concentration (CMC), the washing effect increases sharply. Therefore, the concentration of detergent in the solvent should be higher than the CMC value to have a good washing effect. However, when the concentration of surfactant is higher than the CMC value, the incremental increase in washing effect is not obvious and it is not necessary to increase the concentration of surfactant too much.
When removing oil by solubilization, the solubilization effect increases with increasing surfactant concentration, even when the concentration is above CMC. At this time, it is advisable to use detergent in a local centralized manner. For example, if there is a lot of dirt on the cuffs and collar of a garment, a layer of detergent can be applied during washing to increase the solubilizing effect of the surfactant on the oil.
②Temperature has a very important influence on the decontamination action. In general, increasing the temperature facilitates the removal of dirt, but sometimes too high a temperature can also cause disadvantages.
The increase in temperature facilitates the diffusion of dirt, solid grease is easily emulsified at temperatures above its melting point and the fibers increase in swelling due to the increase in temperature, all of which facilitate the removal of dirt. However, for compact fabrics, the microgaps between the fibers are reduced as the fibers expand, which is detrimental to the removal of dirt.
Temperature changes also affect the solubility, CMC value and micelle size of surfactants, thus affecting the washing effect. The solubility of surfactants with long carbon chains is low at low temperatures and sometimes the solubility is even lower than the CMC value, so the washing temperature should be raised appropriately. The effect of temperature on the CMC value and micelle size is different for ionic and non-ionic surfactants. For ionic surfactants, an increase in temperature generally increases the CMC value and reduces the micelle size, which means that the concentration of surfactant in the washing solution should be increased. For non-ionic surfactants, an increase in temperature leads to a decrease in the CMC value and a significant increase in micelle volume, so it is clear that an appropriate increase in temperature will help the non-ionic surfactant to exert its surface-active effect. However, the temperature should not exceed its cloud point.
In short, the optimum washing temperature depends on the detergent formulation and the object being washed. Some detergents have a good detergent effect at room temperature, while others have a much different detergency between cold and hot washing.
③ Foam
It is customary to confuse foaming power with washing effect, believing that detergents with high foaming power have a good washing effect. Research has shown that there is no direct relationship between the washing effect and the amount of foam. For example, washing with low foaming detergents is no less effective than washing with high foaming detergents.
Although foam is not directly related to washing, there are occasions when it helps to remove dirt, for example, when washing dishes by hand. When scrubbing carpets, foam can also take away dust and other solid dirt particles, carpet dirt accounts for a large proportion of dust, so carpet cleaning agents should have a certain foaming ability.
Foaming power is also important for shampoos, where the fine foam produced by the liquid during shampooing or bathing leaves the hair feeling lubricated and comfortable.
④ Varieties of fibers and physical properties of textiles
In addition to the chemical structure of the fibers, which affects the adhesion and removal of dirt, the appearance of the fibers and the organization of the yarn and fabric have an influence on the ease of dirt removal.
The scales of wool fibers and the curved flat ribbons of cotton fibers are more likely to accumulate dirt than smooth fibers. For example, carbon black stained on cellulose films (viscose films) is easy to remove, while carbon black stained on cotton fabrics is difficult to wash off. Another example is that short-fiber fabrics made of polyester are more prone to accumulate oil stains than long-fiber fabrics, and oil stains on short-fiber fabrics are also more difficult to remove than oil stains on long-fiber fabrics.
Tightly twisted yarns and tight fabrics, due to the small gap between the fibers, can resist the invasion of dirt, but the same can also prevent the washing liquid to exclude the internal dirt, so tight fabrics start to resist dirt good, but once stained washing is also more difficult.
⑤ Hardness of water
The concentration of Ca2+, Mg2+ and other metal ions in the water has a great influence on the washing effect, especially when the anionic surfactants encounter Ca2+ and Mg2+ ions forming calcium and magnesium salts which are less soluble and will reduce its detergency. In hard water, even if the concentration of surfactant is high, the detergency is still much worse than in distillation. For the surfactant to have the best washing effect, the concentration of Ca2+ ions in the water should be reduced to 1 x 10-6 mol/L (CaCO3 to 0.1 mg/L) or less. This requires the addition of various softeners to the detergent.
Post time: Feb-25-2022



